Most of the Elements in Groups 16-18 Are Classified as
A Hydrogen Oxygen Helium B Oxygen Carbon Argon C Nitrogen Oxygen Argon D. 1 Metals 2 Metalloids 3 Non- metals.

Inspirationallegal Electron Configuration Practice Worksheet Answer Key Check More At Htt Counting Atoms Worksheet Practices Worksheets Electron Configuration
Anomalous Behaviour of Fluorine.
. Apart from water these materials are all toxic foul-smelling gases. Anomalous Behaviour of Oxygen. Elements are Classified Basically Into Three Categories.
As a result these groups contain non-metals like nitrogen oxygen and sulfur. Most of the elements in the periodic table are classified as metals. Nuclear charge is decreasing.
Anomalous Behaviour of Oxygen. In chemistry and atomic physics the main group is the group of elements sometimes referred to as representative elements whose lightest members are represented by helium lithium beryllium boron carbon nitrogen oxygen and fluorine as. Sulphur - Allotropic Forms.
9 circ C respectively. As the group 1 elements of the periodic table are considered from top to bottom the first ionization energy of each successive element decreases. Learn vocabulary terms and more with flashcards games and other study tools.
They show decreasing thermal stability with increasing relative atomic mass of X. See answer 1 Best Answer. The thing they have in common is the progressive filling of a p-orbital.
One reason for this is that the a. Let crack it. The elements of group 16 combine with a wide range of other elements and the bonding is largely covalent.
What are the four groups of elements as classified by the ancient Greeks. Enery required to remove an electron from a gaseous atom. Review Questions 2 Groups 16 17 and 18 1What are the 3 most prevalent gases in our atmosphere from most prevalent to least prevailed.
Atom or bonded group of atoms that has a positive or negative charge. - 3 coordinate bonds PX3. Concept of Group 16 Elements.
Start studying Periodic Table. You just studied 16 terms. This session include Multiple choice questions on group 16 17 18 elements.
Now up your study game with Learn mode. Concept of Group 15 Elements. Most common starting material for synthesising P compounds.
These are mild Lewis bases halogen substituents are electronegative so worse electron donors than PH3. Occurrence of Elements of Groups 16 17 and 18. Which energy level of the period 4 transition elements is.
Metallic elements share some similar properties while nonmetallic elements have their own set of properties. The inert gas present in the second long period is Xe. Phosphorus - Allotropic Forms.
In one hour we will solve maximum 30 mcq with proper explanation. Most elements in groups 16 through 18 are classified as. The boiling points of neon and krypton are -245.
Groups 16 - 18. 9 circ C and -152. - 5 coordinate bonds PX5.
There is total 118 elements. Atomic and Physical Properties of Elements of Group 16 17 and 18. The elements all form hydrides of the type XH 2.
Groups 13-16 dont really fit together in any nice way so lets look at them one by one. Enery level of the period 4 transition elements is being filled with electrons. Statement that atoms tend to gain lose or share electrons to acquire a full set of 8 valence electrons.
Which three groups of the Periodic Table contain the most elements classified as. Most of the elements in group 16 through 18 are classified as. Distance between the valence electron and the nuceus is increasing d.
Electronic Configuration of Elements of Group 16 17 and 18. The question contain these choicesA groups 1 16 and 18B groups 5 7 and 9C groups 2 3 and 4D groups 15 16 and 17The correct answer is C groups 2 3 and jessiesavannah jessiesavannah 03282018 Chemistry College Which three groups of the periodic table contain the most elements classified as transition elements 2. The group 16 elements of modern periodic table consist of 5 elements oxygen sulphur selenium tellurium and polonium.
The elements in this group are also known as the chalcogens or the ore-forming elements because many elements can be extracted from the sulphide or oxide ores. Number of neutrons is decreasing c. Groups 1 2 13 14 15 16 17 and 18.
P forms stable halides with group 17 elements. Non-basic oxidation resistant but prone to hydrolysis. The metallic properties of these elements decrease along each period and increase down each group.
About 70 percent of the elements are metals while the remaining 30 are nonmetals. The hydrides dissolve in water to give. Using these data estimate the boiling point of argon.
Number of principal energy levels is decreasing b.
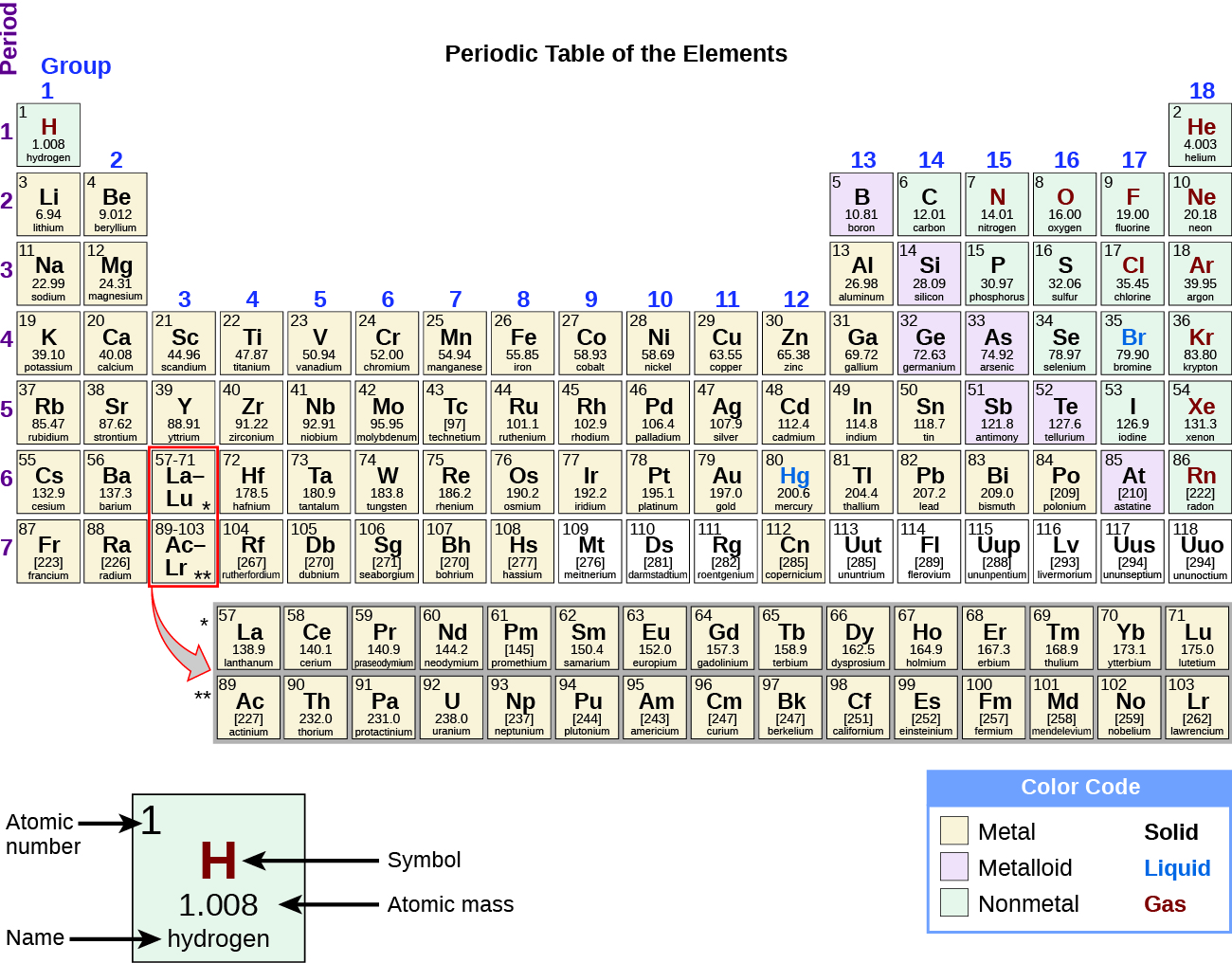
2 5 The Periodic Table Chemistry
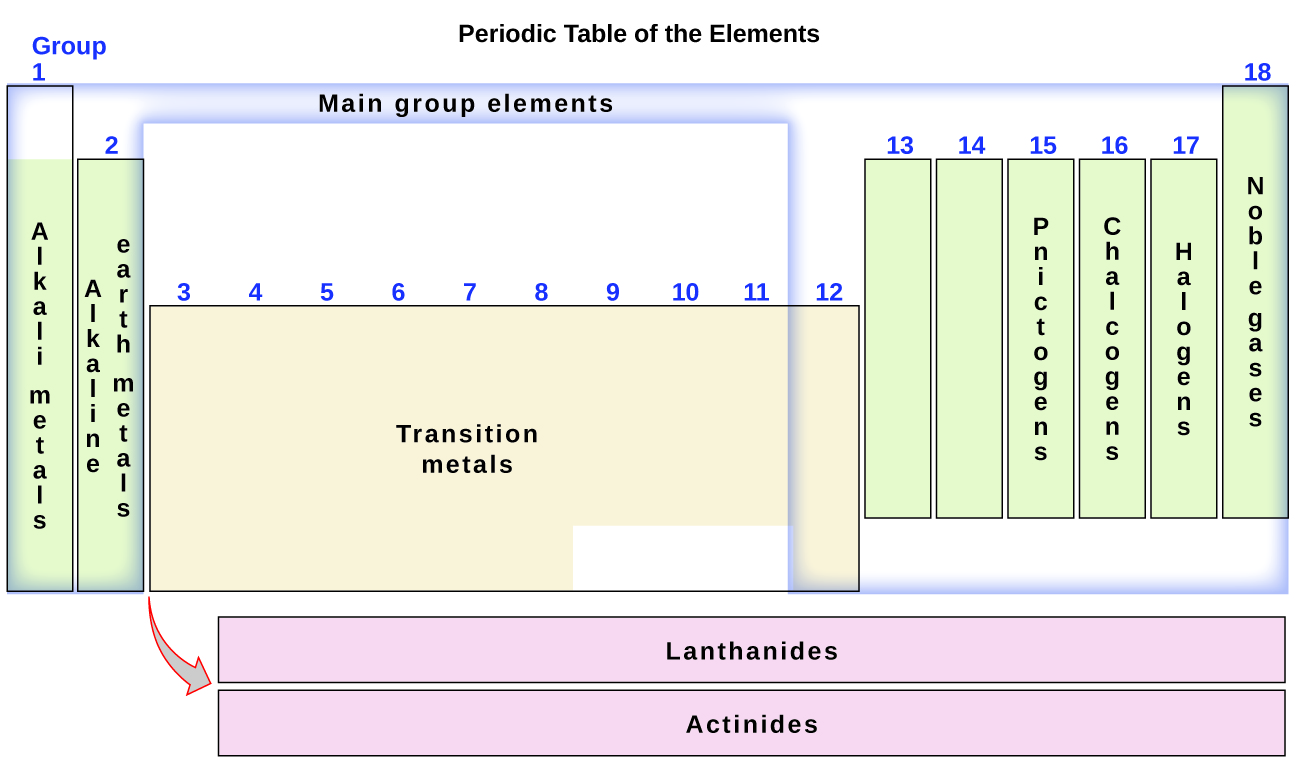
2 5 The Periodic Table Chemistry

2 5 The Periodic Table Chemistry

Periodic Table Elements Explained Teaching Chemistry Apologia Chemistry Learning Science
No comments for "Most of the Elements in Groups 16-18 Are Classified as"
Post a Comment